The Structures – The Problems
Xypex products play a key role in the waterproofing and protection of concrete against water penetration, chloride ion attack, cracking, carbonation, sulphate attack, Alkali Aggregate Reaction and freeze/thaw damage – problems typically associated with the reduced service life of marine structures.
Water Permeability & Corrosion
The primary purpose of waterproofing concrete marine structures is the protection of reinforcing steel from the damaging effects of corrosion. The nature of concrete and the problems associated with placement and consolidation means having to deal with permeability issues permitting the penetration of water into the substrate and through to the reinforcing steel. With the presence of oxygen this can initiate corrosion.
This permeability facilitates the entry and diffusion of chlorides into contact with the reinforcing steel. The resulting loss of alkalinity and hence the passivating layer allow for an electrochemical process culminating in corrosion of the reinforcing steel and the expansive disruption of the concrete substrate.
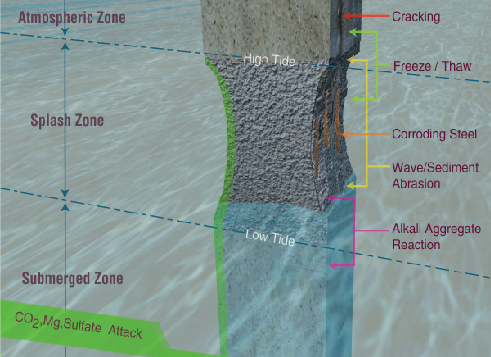
Cracking
Cracks in the concrete are the most obvious means by which water and damaging chemicals can enter a structure. These cracks are formed in a number of different ways but the most common are drying shrinkage, thermal cracking, strain formed cracks, settlement cracks and plastic shrinkage cracking in the slab.
Surface Deteroriation
The principal cause of surface degeneration is the impact of silt, sand, gravel and other solids impacting and rolling against the concrete surface. This causes deterioration in the long term, resulting in some form of surface rehabilitation during the service life of the structure.
Sulphate Attack & Alkali Aggregate Reaction
Where sulphates are present in water or soils, the permeability of concrete and the presence of water allows sulphate ions to diffuse into the concrete and create an expansive reaction causing spalling and deterioration.
A similar effect is caused by Alkali Aggregate Reaction whereby the presence of water in concrete permits a reaction between silica in certain aggregates and the alkalis in cement.
Carbonation
Carbon dioxide in the air reacts with calcium hydroxide in the concrete to form calcium carbonate which reduces the alkalinity of the concrete. Below a pH of 10, the rebar’s thin layer of surface passivation dissolves and corrosion of the reinforcing steel takes place at an accelerated rate.